Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus eget nunc nisi. Proin tristique semper tempus. Nullam lobortis augue nec ligula congue, eget malesuada dolor condimentum.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus eget nunc nisi. Proin tristique semper tempus.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus eget nunc nisi. Proin tristique semper tempus.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus eget nunc nisi. Proin tristique semper tempus.
DI Quality Certification Services Pvt. Ltd. can support your organization to improve organizational performance and mitigate risks via ISO 13485 and applicable regulatory standards.

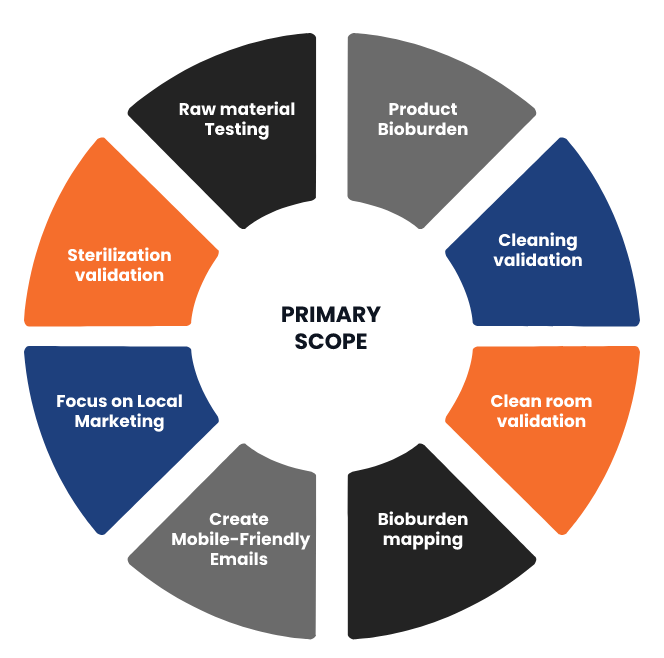
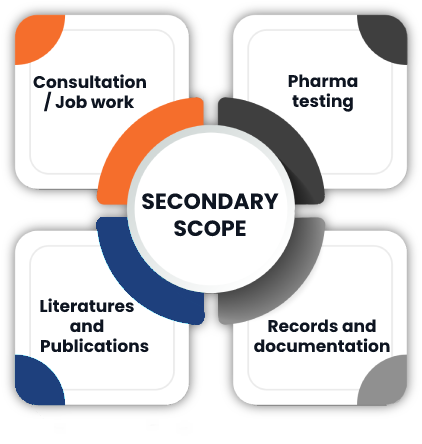
Second Floor of the premises Plot no A-28,
New Door No:18, 86th Street, 18th Avenue,
Ashok Nagar, Chennai – 600083,
Tamil Nadu , India
2nd Floor, office number
232,
VTP Trade Park, Katraj-Hadapsar Road,
Undri, Pune-411060,
Pune , India